The overarching goal of our research is to define how epithelialized organ-specific biology responds to noxious stimuli such as microbes and chemical irritants. For example, urothelial cells express uroplakins, and through these tissue-restricted surface molecules may interact with microbes in a unique fashion. We use both bacterial and parasitic urinary tract infections as models of study.
We have also employed nitrosamines, a bladder irritant and carcinogen for humans as well as mice, to understand how urothelial cells respond to noxious stimuli. This work was published, in close collaboration with Phil Beachy's lab, in Nature Cell Biology. One of the most striking findings from this study was the effects of nitrosamines on clonality of the bladder urothelium. We examined clonality of the bladder urothelium through use of "Rainbow" mice, which initially express EGFP but upon exposure to Cre recombinase activity can recombine to express any of three additional distinct fluorescent proteins in a stochastic fashion. Rainbow mice induced to express Cre recombinase (under the control of an actin promoter) demonstrate even distribution of all four fluorescent proteins in the urothelium:
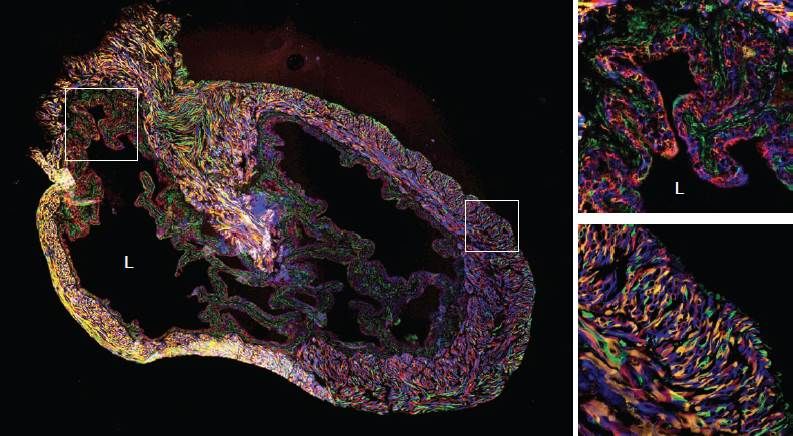
"L" marks the bladder lumen. Small inset squares in left panel are shown as high power fields in the right panels. The top and bottom right panels demonstrate multicolor labeled urothelium and bladder stroma, respectively. Adapted from Shin et al.
When Rainbow mice are induced to express Cre recombinase and are then given carcinogenic nitrosamines, the resulting carcinoma in situ (a preinvasive form of cancer) is monoclonal or oligoclonal, based on urothelial expression of only one or two fluorescent proteins:
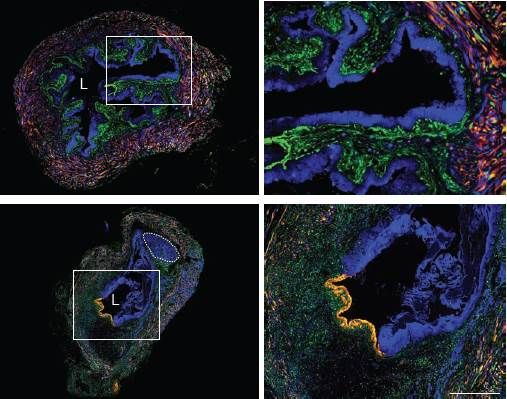
"L" marks the bladder lumen. Small inset squares in left panels are shown as high power fields in the right panels. The top and bottom right panels demonstrate monoclonal (blue cells) and oligoclonal (blue and yellow) expansion of the urothelium, respectively. Adapted from Shin et al.
The oligoclonality of nitrosamine-induced bladder cancers was preserved even in advanced, invasive tumors, helping to lay rest the long-standing question regarding whether bladder cancers arise from one or multiple initial cancerous cells:
"L" marks the bladder lumen. Small inset squares in left panels are shown as high power fields in the right panels. The top and bottom right panels demonstrate monoclonal (blue cells) and oligoclonal (blue and yellow) expansion of the urothelium, respectively. Adapted from Shin et al.
The oligoclonality of nitrosamine-induced bladder cancers was preserved even in advanced, invasive tumors, helping to lay rest the long-standing question regarding whether bladder cancers arise from one or multiple initial cancerous cells:
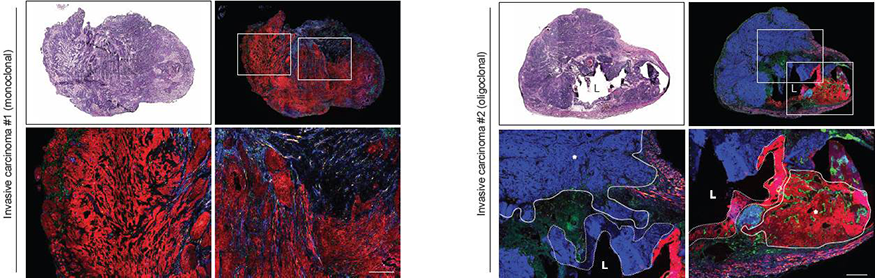
Bladders from Rainbow mice induced to express Cre recombinase and exposed to nitrosamines for 6 months were analyzed by standard histologic staining (top left panels) or for four color fluorescence (top right panels). Bottom panels show magnified views of the regions highlighted by white boxes in top right panels. Carcinomas within a single bladder arise from one (left set of panels, monoclonal) or several (right set of panels, oligoclonal) cells, respectively. In the right set of panels, tumors are outlined by solid lines with asterisks and nearby carcinoma in situ regions are outlined by dotted lines. L, bladder lumen. Adapted from Shin et al.
This set of findings could be a game changer in terms of therapeutic and diagnostic approaches to bladder cancer. Previously, it has not been clear whether bladder cancers arise as the result of cancerous mutations in many cells in the bladder lining due to ongoing exposure to toxins excreted in the urine, or if it is due instead to a defect in one cell or cell type. A better understanding of how bladder cancers begin and progress may enable targeting the cancer stem cell, or the identification of molecular markers that would facilitate earlier diagnosis and disease monitoring.
Epifluorescent imaging of Schistosoma haematobium eggs in the mouse bladder.
Our past and current grant support:







Stanford Child Health Research Institute
Stanford Center for Clinical Human Immunology
Stanford Institute for Stem Cell Biology and Regenerative Medicine