Traditional diagnosis of intestinal schistosomiasis has relied on the Kato-Katz method, where stool smears on slides are stained and schistosome eggs are manually counted under the microscope. For urogenital schistosomiasis, traditional diagnosis has depended on syringe filtration of urine samples followed by manual microscope-based counting of eggs retained on the filters.
These techniques are slow, labor-intensive, and require a microscope, slides, and other infrastructure that can be challenging to install in the field. An emerging point-of-care diagnostic that may be alternative to Kato-Katz is urine circulating cathodic antigen (CCA). Urine CCA is proving to be highly sensitive for detecting intestinal schistosomiasis, but it often fails to detect low intensity infections, and is poor for diagnosing urogenital schistosomiasis (reviewed by Silveira and our group in this paper and Le and Hsieh in this paper).
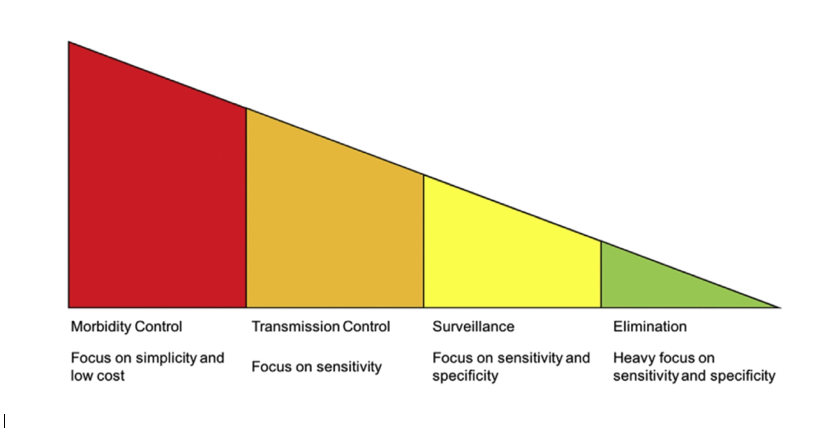
Indeed, not only is there a need for better schistosomiasis diagnostics more broadly, there are differing needs for such diagnostics according to each endemic country’s stage of schistosomiasis control:
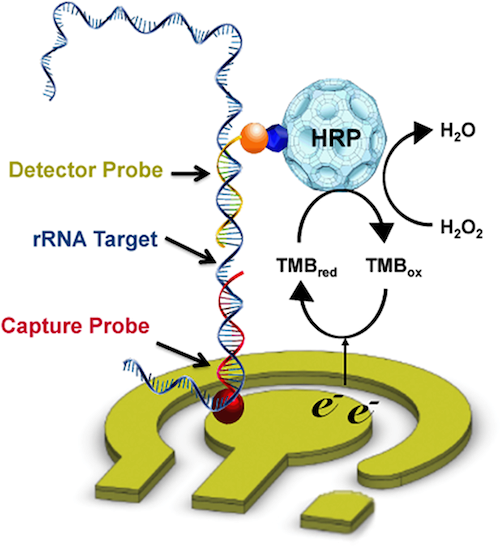
We have worked towards improving schistosomiasis diagnostics through several approaches. One technology we have explored is the use of electrochemical sensors (Mach et al., PLOS NTD 2015). Specifically, we designed and tested pairs of capture and detector probes predicted to bind ribosomal RNA from Schistosoma haematobium eggs. The signal consists of a redox reaction driven by horseradish peroxidase (HRP) conjugated to the detector probe. The current associated with the reaction is detected, signifying a specific oligonucleotide interaction has occurred:
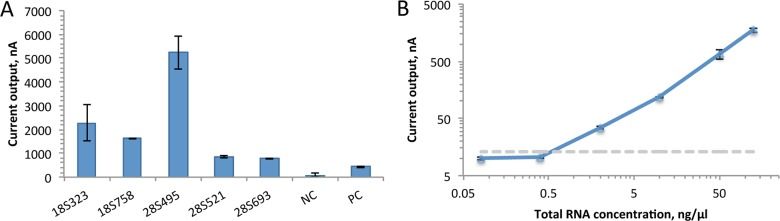
We tested a number of candidate capture and detector probes, and after choosing the best-performing set, we measured its limit of detection by titrating S. haematobium ribosomal RNA:
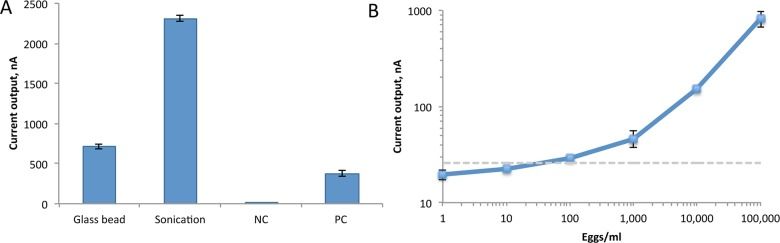
Our next step was to determine if it was better to lyse S. haematobium eggs in human urine by agitation with glass beads or sonication. We found that sonication worked better, which lead us to determine the limit of detection of sonicated eggs in urine:
Although this technology is promising, the instrumentation is not yet point-of-care, and sample prep remains to be optimized.
We have also been exploring microfiltration technology as a means by which to improve schistosomiasis diagnosis. In this paper we fabricated a microfiltration device consisting of a reservoir, syringe pump, inlet and oulet, and a trapping array of trumpet-shaped PDMS wells:

Computational fluid dynamics simulations allowed us to model how the eggs would be trapped by the array:
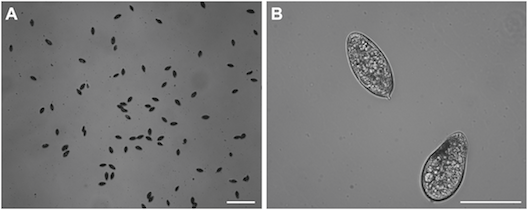
These simulations facilitated calculation of magnitude of egg velocity during trapping:
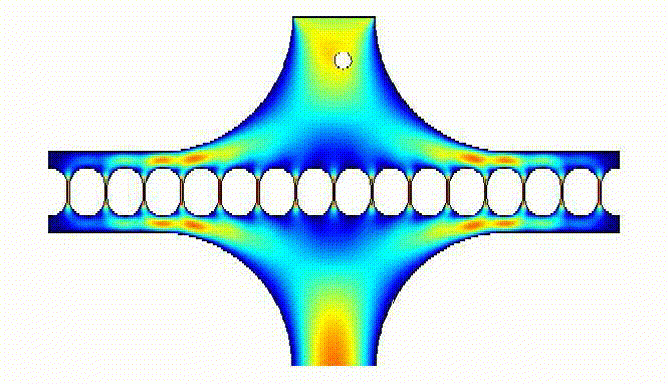
They also allowed us to determine flow resistance and sequential loading of eggs in the array wells:
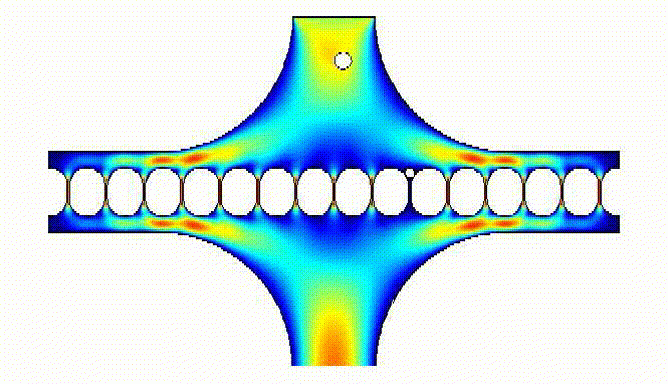
Large numbers of eggs could be trapped by our device:
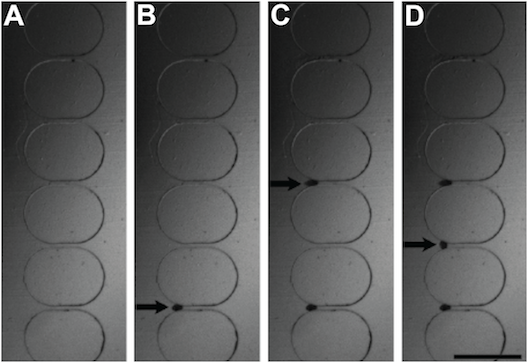
These images show time lapse photography of the eggs loading onto the wells:
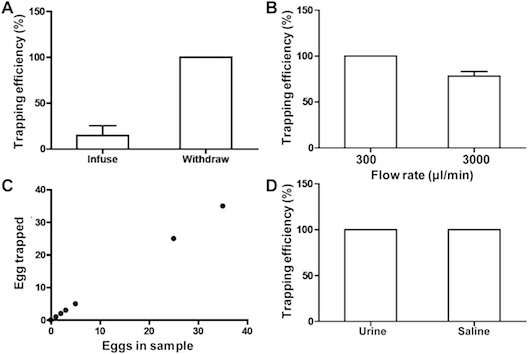
We were able to determine the trapping efficiency of our device according to flow rate, numbers of eggs loaded, whether the eggs were in saline or urine, and whether eggs were infused or aspirated:
We are also developing more rapid and accurate diagnostics for urinary tract infections.